What is pH level?
pH is a fundamental property of a solution that indicates whether it is acidic, neutral, or basic. In chemistry, and especially in titration, pH plays a crucial role in determining reaction progress and identifying the equivalence point.
The pH value measures the concentration of hydrogen ions (H⁺) in a solution. Here, ‘p’ stands for power and ‘H’ for hydrogen—together meaning the power of hydrogen. This measurement helps categorize solutions as acidic, neutral, or basic and directly influences how they behave during acid–base titration experiments.
So, why is pH important? It regulates nutrient availability, biological functions, microbial activity, and the chemical behavior of a solution. In titration specifically, pH helps detect the point of neutralization, guides indicator selection, and ensures accuracy in chemical analysis.
What is the role of pH in titration?
-
It determines how the titration curve develops.
-
It signals the equivalence point where the reaction is complete.
-
It assists in selecting the correct indicator for accurate results.
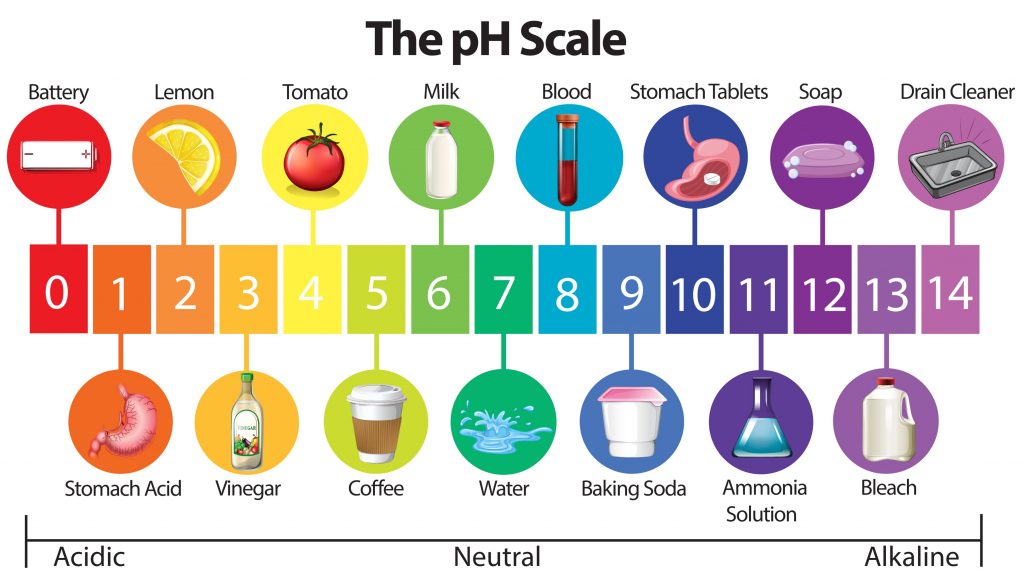
pH Scale and Titration- The Acids, The Bases, and The Neutral
The pH scale is a 14 pointer scale. Having a pH less than 7 will classify a solution as acidic. For example, lemon juice or citric acid has a pH of 2 making it a highly acidic solution. Similarly, having a pH over 7 will land a solution in the base category like bleach, with a pH of 12. There is however a balance or a neutral pH value, this suggests that the solution is neither acidic nor basic in its nature.
 |
How can one identify the pH levels of a solution?
The Litmus Test
- There are several ways in which one can identify the pH levels of a solution, the most common and easiest being the ‘Litmus Test’. The test essentially uses a strip of paper that is made from lichens. This strip of paper changes its color based on the characteristics of the solution it interacts with. Light blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions. A neutral litmus test would result in a purple shade.
- pH paper, however, comes with some restrictions. First, it is not an accurate indicator of pH and does not give us a numerical pH value. Instead, it roughly indicates the nature of the sample.
pH Meter
- Another way is the pH meter, which is a much more articulate and accurate method of testing. A pH meter is a scientific apparatus that can not only identify if a solution is acidic or alkaline but also give us an exact measure of its pH levels.
How is a pH test relevant and important?
- One of the common usages of these pH tests and indicators is acid-base titration. A process of quantifying the concentration of acid or base in a solution by neutralising it with the exact same proportion of base or acid.
- In situations like these where one wants to know the exact value, digital pH indicators have proven to be the preferred choice.
- The titration of a strong acid – strong base is a method used to determine the concentration of the acidic solution by titrating it with a basic solution of known concentration, or vice-versa. When a strong acid reacts with a strong base, they completely neutralise each other, this results in the residue of salt and water.
- A weak acid and strong base or vice-versa make the titration curve irregular and the pH shifts at a slower rate with small additions of titrant near the equivalence point.
Different industries, different uses
pH monitoring is essential in several industries, like wastewater treatment, aquaculture, pharmaceuticals, food and beverages, paper, and the textile industries. Each industry has a specific pH level prescribed for their products to remain safe for use.
-
Wastewater
 |
While treating wastewater, several acidic substances are removed from the water. The pH levels are adjusted with the use of chemicals and are monitored at every phase of the treatment so that the treated water is safe and fit to reuse once the process is complete.
-
Aquaculture
 |
In the aquaculture industry, it becomes crucial for the industry to maintain the right pH levels to ensure safe and consumable seafood. Therefore, in this case too, the pH category level is monitored very closely.
-
Food and Beverage
 |
Just like the aquaculture industry, the food and beverage industry is also an industry that keeps a close check on the pH levels at different stages of manufacturing. Observing the pH levels of bottled water and meat products is a common and encouraged practice.
-
Paper and Textile
 |
Paper and textile industries produce large quantities of wastewater that can be harmful to their equipment and the environment. So, it’s their moral responsibility to keep an eye on the pH levels of this wastewater.
Keeping a close eye on the pH levels
All these industries need to very closely monitor and maintain their desired pH levels which makes it important to use an apparatus that is accurate and eliminates chances of human errors.
Microlit E-Burette helps deliver precise samples and guarantees accuracy to industrial titrations. This can be credited to its state-of-the-art design and precision. It exhibits excellent chemical compatibility and helps in performing precise titrations with reliability in practical laboratory environments.
To know more about the Microlit E-Burette, you can write to us at info-usa@microlit.com.
Some Frequently Asked Questions
1. What is the role of pH in titration?
The pH in titration determines the point at which the acid and base have completely reacted, known as the equivalence point. Monitoring pH helps chemists select the right indicator and ensures accurate results. In other words, pH acts as the guiding factor in identifying the completion of titration.
Pro Tip: Using advanced digital burettes and titration systems from Microlit makes pH monitoring more precise and reliable.
2. Why is pH adjusted to 10 during titration?
In certain titrations, such as EDTA complexometric titrations, the pH is adjusted to around 10 to ensure the metal ions form stable complexes with the reagent. At this pH, the reaction becomes more selective and accurate, minimizing side reactions.
3. How do pH indicators change color during titration?
pH indicators are weak acids or bases that change color depending on the hydrogen ion concentration of the solution. During titration, as the pH shifts, the indicator transitions between colors, signaling that the equivalence point has been reached.
4. What happens to pH during titration?
During titration, the pH changes gradually and then shows a sharp shift near the equivalence point. For example, in a strong acid-strong base titration, the pH moves slowly at first, then rises rapidly as the reaction nears completion. This sharp change makes it possible to identify the endpoint.




 33986
33986