One of the most common burette techniques of quantitative analysis in the healthcare and life sciences industry the world over is titration. It is a routine laboratory task that is typically taught while studying at a school or a university or while training and finds application in a number of industries including F&B, pharma, water treatment, among many more. The following narrative discusses titrations and elaborates on their manifold uses and benefits.
What is titration?
Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete.
technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete.
Often, a visual indicator (buffer or pH solution) is used to signal the completion (end-point). Since the volume of the titrant is already known, one can easily determine the concentration of the analyte, using the formula of titration.
There are many types of titrations with different processes and objectives. The four widely used ones include – acid-base titrations, redox titrations, complex metric titrations and precipitation titrations.
Detecting the end-point of a titration
End-point is a physical change that indicates the completion of titration. It is a signal for the user to stop adding any more titrant to the analyte. It is usually indicated by some form of a visual indicator like a color change, a visible precipitate, or a change on an electronic readout.
Performing a routine titration to determine the concentration of an analyte
The following steps employ a conventional glass burette to perform a titration. The same experiment can also be carried with the help of an electronic burettes, a better alternative that minimizes the risk posed by multiple sources of error that might occur in titrations with conventional glass burette.
- Place a known quantity (volume) of the solution of unknown concentration (the analyte) in an Erlenmeyer flask and fill the solution of known concentration (the titrant)in the burette.
- Open the tap of the burette gradually and let the titrant trickle down into the analyte. Determine the correct titration speed – too slow, and the titration period is unnecessarily prolonged; too fast, and the equivalence point is exceeded.
- Keep an eye on the colour of the analyte – the completion of a titration is usually indicated by a colour change.
Lastly, if the titrant and the analyte are in a 1:1 mole ratio, use the following equation to determine the concentration of the analyte.
Mtitrant x Vtitrant = Manalyte x Vanalyte
where,
Mtitrant = Molarity (M) of the titrant,
Vtitrant = Volume (V) of the titrant,
Manalyte = Molarity (M) of the analyte, and,
Vanalyte = Volume (V) of the analyte.
Note: Molarity is the concentration of a solution expressed as the number of moles of solute per liter of solution.
What is back titration?
Back Titration, also called indirect titration, is a titration done in reverse. Typically employed in acid-base titrations, back titration is used when the concentration of an excess reactant is known, but the concentration of the analyte needs to be found out. In this case:
- Firstly, the concentration of the analyte is determined by reacting it with a known amount of the excess reagent.
- The remaining excess reagent is then titrated against a second reagent, the result of which indicates how much of the excess reagent was used in the first titration.
- This facilitates the easy calculation of the concentration of the original analyte.
Back titration is useful in cases where the reaction between the analyte and the titrant occurs very slowly, or when the analyte is in a non-soluble solid state.
MICROLIT E-BURETTE helps in performing precise and accurate titrations
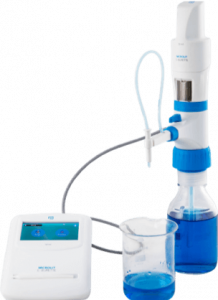
MICROLIT E-BURETTE is a state-of- the-art Motor Operated Burette. It is commonly referred to as a Digital Burette or Electronic Titrator.
Designed with ergonomics and intuitive liquid handling in mind, MICROLIT E-BURETTE is widely used in industries like Pharmaceutical, Environmental Monitoring and Food & Beverages. It exhibits excellent chemical compatibility and helps achieve precision with reliability in practical laboratory environments. It has the following key features:
1. 3 Pre-set Speeds for Dispensing
2. Motor Controlled Piston Movement
3. No wastage of reagents during purging with Recirculation Mode
4. Easy Dispensing with horizontal & vertical adjustment of the delivery nozzle
5. Quick Titrations with TFT Touchscreen
6. In-built memory slots for quick reference
For more information about Microlit E-Burette, visit: https://www.microlit.us/product-category/burette/.




 10861
10861





