Fundamentals of Calibration
The purpose of calibrating any device is to verify its functionality. The process is generally carried out via a standardized procedure and varies with every lab equipment. In case of pipettes, calibration is performed to ensure that it accurately dispenses the expected liquid volume.
Difference between ISO 8655 and ISO 17025
ISO 8655 is an international standard designed for performing calibration and testing of piston-operated volumetric equipment, such as pipettes, dispensers, precision laboratory syringes, burettes and dilutors. It was drafted by the International Organization for Standardization’s (ISO) Technical Committee 48, in order to complement the ISO 17025 standard. ISO 8655 is a very specific and highly detailed set of standards that a pipette manufacturer must adhere to, for accurately calibrating pipettes. It defines the necessary methods, test equipment, reporting requirements and test conditions for pipette calibration. Currently, ISO 8655 is the most important ISO standard used for the calibration of piston-operated pipettes, dispensers and burettes.
ISO 17025 is a quality management system, serves as the primary standard for testing and calibration facilities. While ISO 17025 and ISO 9000 have many similarities, ISO 17025 assesses technical proficiency in lab testing and calibration services and is applicable to organizations that generate testing and calibration results.
On the contrary, ISO 17025 standard does not have any requirements related to the quality of processes. In addition, it does not point to any specific guidelines for a device, but applies to a variety of equipment, such as medical devices, weight scales, electronic devices, pressure meters, calipers and communication equipment.
Detailed Overview of ISO 8655
ISO 8655:2022 is the latest version ISO 8655 standard. Currently, the ISO 8655:2022 standard comprises of total nine parts, as compared to seven parts in the previous version.
- Part 1: Terminology, general requirements and user recommendations
- Part 2: Pipettes
- Part 3: Burettes
- Part 4: Dilutors
- Part 5: Dispensers
- Part 6: Gravimetric reference measurement procedure for the determination of volume
- Part 7: Alternative measurement procedures for the determination of volume
- Part 8: Photometric reference measurement procedure for the determination of volume
- Part 9: Manually operated precision laboratory syringes
For pipette calibration, ISO 8655 standard defines requirements for air displacement pipettes, as well as positive displacement pipettes, including single channel and multi-channel pipettes. It covers both fixed and adjustable volume pipettes, as well as automated and manual pipettes.
The ISO 8655 standard strictly describes six elements that are required for precise, reproducible, ISO-compliant measurements:
- Lab and Environmental Conditions
- Methodology
- Process Requirements
- Measurement Specifications
- Acceptable Measurement Uncertainties
- Maximum Permissible errors
Lab and Environmental Conditions
In order to be compliant to ISO 8655, pipette measurements have to be performed in an extremely controlled and vibration-free lab environment. Pipette calibrations require continuous monitoring of test conditions for a valid demonstration of performance and measurement analysis.
The environmental and lab conditions that are considered as acceptable are defined below:
- Temperature: Constant (15-30°C) {±0.5°C}
- Relative Humidity: ˃50%
- Altitude: Ground Level
- Air Flow: Draft Free
- Evaporation Rate: ~0
- Vibration: ~0
- Static: ~0
Methodology
Currently, there are three parts dedicated to different testing techniques, including two reference methods, gravimetric and photometric, as well as a new part defining alternative methods. As per ISO 8655, the main methodology that should be used for the calibration of piston-operated pipettes and other related instruments is gravimetric measurement analysis.
Process Requirements
The new ISO is designed to consider the pipette tip and the instrument as one whole system. It details a step-wise process for producing reliable measurement data. These processes include suitable technique for pipette tip attachment, pre-wetting, aspiration and dispensing.
Measurement Specifications
One notable addition in the latest ISO is that the service provider must change the pipette tip at least once per measured volume. Further, the standard comprises of information on evaporation rate determination, measurement container requirements, time lapse for test completion and minimum number of measurements required.
Acceptable Measurement Uncertainties
Measurement uncertainty is described as a measured value that represents the level of ‘doubt’ or possible error in the calculation of calibration caused by from external influences. This can vary based on the quality and controls maintained during a calibration test. ISO accredited laboratories must verify, estimate and report measurement uncertainties for each calibration.
Maximum Permissible Errors
ISO 8655 clearly defines the Maximum Permissible Limits for piston-operated pipettes The standard describes the maximum permissible random error, as well as maximum permissible systematic error limits for a device at different volumes between 1-10,000uL. These error values are twice in case of multichannel pipettes. Typically, pipette manufacturers’ specifications are well within these error limits, however, for low volumes, conforming to ISO 8655 error limit can be challenging for many manufacturers.
ISO 8655 and FDA Audits
FDA audits all drug development labs regularly, which helps in ensuring proof of efficacy, as well as data integrity. Therefore, manufacturers need to provide quality assurance validation to avoid any regulatory issues. ISO 8655 compliance is indispensable for a pipette manufacturer as it is mandatory to prove the accuracy and reliability of a pipette.
FDA audits, performed for drug development quality control also require an ISO 17025 accreditation audit to ensure the integrity of a pipette calibration service provider. But, when the pipette calibration service provider adheres to ISO 8655 standards and controls, the auditor evaluates the standards as ‘more than expected’. For pipette manufacturers, conforming to ISO 8655 standards helps to showcase service integrity, which in turn, corroborates the trust and reliability of the drug development lab.
Microlit Products conform to ISO 8655 and ISO 17025
We take pride in producing cutting-edge lab instruments that conform to both ISO 8655 and ISO 17025 requirements, including bottle top dispensers, micropipettes, and burettes. Microlit products are extremely accurate and ergonomically designed, resulting in a remarkable user experience and flawless precision in the real laboratory environment.
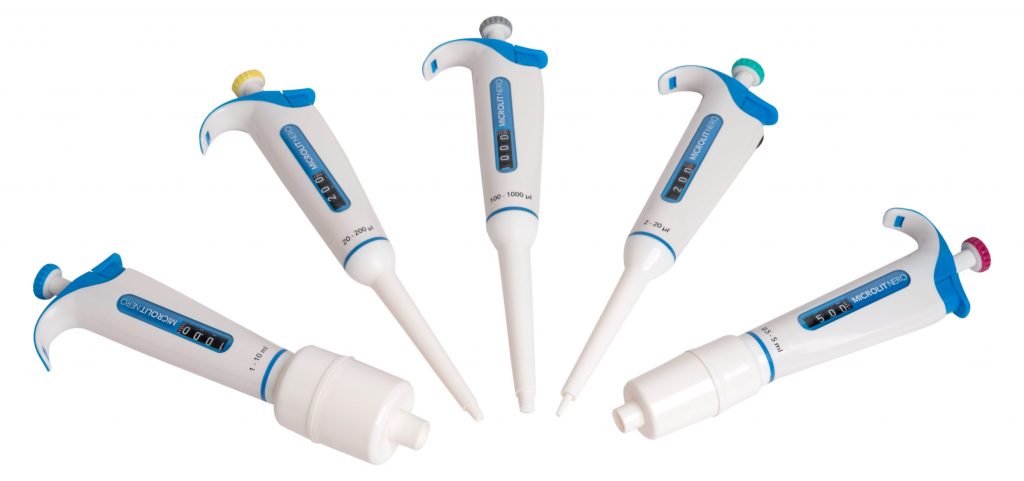
Our newest product, Microlit NERO, now has our patented UniCal™ technology, a one-time calibration mechanism that enables quick in-lab calibration without enabling the digits to be disengaged from the plunger mechanism.
To know more about Microlit products, visit our products page or email us at info-usa@microlit.com.




 9844
9844